Dosing for CABOMETYX® (cabozantinib) with OPDIVO® (nivolumab)
Dosing for CABOMETYX® (cabozantinib) with OPDIVO® (nivolumab)
On This Page:
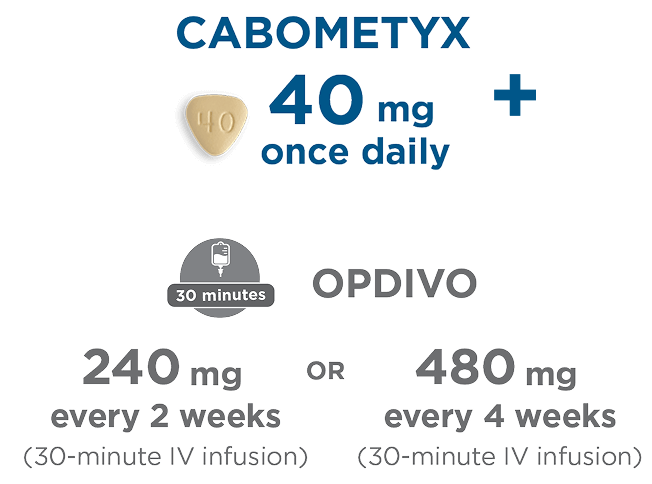
CABOMETYX 40-mg, once-daily starting dose—optimized for combination treatment with OPDIVO1
2 ways to deliver CABOMETYX + NIVOLUMAB in 1L aRCC1,2

An oral and intravenous option grounded in clinical evidence1
OR

An oral and 3- to 5-minute subcutaneous option that may streamline administration for patients1,2*

*Individual results may vary.
Treatment with CABOMETYX should be continued until disease progression or unacceptable toxicity.1
Treatment with OPDIVO should be continued until disease progression or unacceptable toxicity for up to 2 years.1
Recommended administration of CABOMETYX1
Administer on an empty stomach
Administer CABOMETYX at least 1 hour before or at least 2 hours after eating
Swallow CABOMETYX tablet whole
Do not crush, chew, or split CABOMETYX tablets
- Withhold CABOMETYX for at least 3 weeks prior to elective surgery, including dental surgery. Do not administer CABOMETYX for at least 2 weeks after major surgery and until adequate wound healing is observed, to reduce the risk of hemorrhage
- DO NOT substitute CABOMETYX tablets with cabozantinib capsules
- Modify the dose for certain patients with hepatic impairment and for patients taking drugs known to moderately or strongly induce or strongly inhibit CYP3A4
- When administering CABOMETYX in combination with OPDIVO or OPDIVO QVANTIG for the treatment of aRCC, refer to the OPDIVO or OPDIVO QVANTIG Prescribing Information
Advise patients of the following, if a dose is missed and the next scheduled dose is:
in less than 12 hours
- DO NOT make up the missed dose
- Take the next dose at the usual time
in 12 hours or more
- Talk to their doctor or nurse
Pharmacokinetics
- The predicted terminal half-life of CABOMETYX is approximately 99 hours
CABOMETYX is a 1-tablet dose even if dose adjustments are needed1
Withhold CABOMETYX for intolerable Grade 2 ARs, Grade 3 ARs, and ONJ. Upon resolution/improvement (ie, return to baseline or resolution to Grade 1) of an AR, reduce the dosage based on the chart below.
|
Combination |
First reduction |
Second reduction |
|---|---|---|
|
|
|
|
|
Combination |
|
|---|---|
|
First |
|
|
Second |
|
-
Tablets shown are not actual size.
- †
-
If previously receiving 20 mg once every other day, resume at same dose. If not tolerated, discontinue CABOMETYX.
Of the patients who needed to reduce their dosage, most required only 1 dose reduction3
| 20 mg once daily | 20 mg once every other day |
|---|---|
| 50.3% | 8.1% |
The mean average daily dose of CABOMETYX was 30 mg/day3
Primary analysis: Discontinuation rate due to ARs for CABOMETYX + OPDIVO was 6%1
|
Permanent discontinuation |
Dose interruption/reduction |
|
|---|---|---|
|
CABOMETYX or OPDIVO |
20% |
83% |
|
CABOMETYX only |
8% |
46% |
|
OPDIVO only |
7% |
3% |
|
CABOMETYX and OPDIVO |
6%‡ |
21%§ |
|
sunitinib2 |
16.9% |
72.5% |
- ‡
-
Due to the same AR at the same time.
- §
-
Due to the same AR at the same time; 6% for both drugs sequentially.
The discontinuation rate of CABOMETYX alone was 8%1
- Permanently discontinue CABOMETYX for Grade 3 or 4 hemorrhage, development of a GI perforation or Grade 4 fistula, acute myocardial infarction or Grade 2 or higher cerebral infarction, Grade 3 or 4 arterial thromboembolic events or Grade 4 venous thromboembolic events, Grade 4 hypertension/hypertensive crisis or Grade 3 hypertension/hypertensive crisis that cannot be controlled, nephrotic syndrome, or reversible posterior leukoencephalopathy syndrome1
For patients being treated with CABOMETYX in combination with OPDIVO1:
- If ALT or AST >3x ULN but ≤10x ULN without concurrent total bilirubin ≥2x ULN, both CABOMETYX and OPDIVO should be withheld until hepatic ARs recover to Grades 0 or 1. Corticosteroid therapy may be considered. Rechallenge with a single medicine or rechallenge with both medicines after recovery may be considered. If rechallenging with OPDIVO, refer to OPDIVO Prescribing Information
- If ALT or AST >10x ULN or >3x ULN with concurrent total bilirubin ≥2x ULN, both CABOMETYX and OPDIVO should be permanently discontinued
When strong CYP3A4 inhibitors cannot be avoided1

Reduce the daily dose of CABOMETYX if concomitant use with strong CYP3A4 inhibitors cannot be avoided.
Resume CABOMETYX at the dose that was used prior to initiating the strong CYP3A4 inhibitor 2 to 3 days after discontinuation of the strong inhibitor.
Examples of strong CYP3A4 inhibitors¶:
- Boceprevir
- Clarithromycin
- Conivaptan
- Grapefruit juice
- Indinavir/ritonavir
- Itraconazole
- Ketoconazole
- Lopinavir/ritonavir
- Nefazodone
- Nelfinavir
- Posaconazole
- Ritonavir
- Saquinavir/ritonavir
- Voriconazole
When strong or moderate CYP3A4 inducers cannot be avoided1

Increase the daily dose of CABOMETYX if concomitant use with strong or moderate CYP3A4 inducers cannot be avoided.
For example, from 60 mg to 80 mg daily or from 40 mg to 60 mg daily, as tolerated
- Do not exceed a daily dose of 80 mg
Resume CABOMETYX at the dose that was used prior to initiating the strong or moderate CYP3A4 inducer 2 to 3 days after discontinuation of the strong or moderate inducer.
Examples of strong CYP3A4 inducers¶:
- Rifampin
- Phenytoin
- Carbamazepine
- St. John’s wort
Examples of moderate CYP3A4 inducers¶:
- Bosentan
- Phenobarbital
- Etravirine
- Dabrafenib
- ¶
-
Examples listed may not be comprehensive.
ALT=alanine aminotransferase; AR=adverse reaction; aRCC=advanced renal cell carcinoma; AST=aspartate aminotransferase; CYP3A4=cytochrome P450 3A4; GI=gastrointestinal; IV=intravenous; ONJ=osteonecrosis of the jaw; RCC=renal cell carcinoma; ULN=upper limit of normal.
References:
- CABOMETYX® (cabozantinib) Prescribing Information. Exelixis, Inc.
- OPDIVO QVANTIG™ (nivolumab and hyaluronidase nvhy) injection, for subcutaneous use Prescribing Information. Bristol Myers Squibb Company.
- Data on file. Exelixis, Inc.


